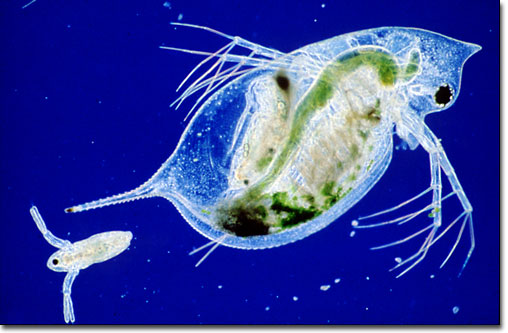
Culture Techniques of Moina : The Ideal Daphnia for Feeding Freshwater Fish Fry 1
R.W. Rottmann, J. Scott Graves, Craig Watson and Roy P.E. Yanong 2
Introduction
Daphnia are small freshwater cladoceran crustaceans commonly called “water fleas.” This common name is the result not only of their size, but their short, jerky hopping movement in water. The genera Daphnia and Moina are closely related. They occur throughout the world and are collectively known as daphnia.
Daphnia have a body consisting of a head and a trunk ( Figure 1 ). The antennae are the main means of locomotion. Large compound eyes lie under the skin on the sides of the head. One of the major characteristics of daphnia is that the main part of the body, the trunk, is enclosed in an external skeleton (carapace). Periodically, they molt or shed their external shell. The brood pouch, where the eggs and embryos develop, is on the dorsal side of the female. In Daphnia, the brood pouch is completely closed, while Moina have an open pouch.
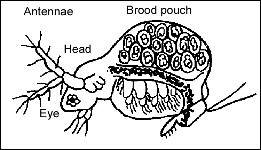 |
| Figure 1. Adult Moina. |
There is considerable size variation between the genera. Moina are approximately half the maximum length of Daphnia. Adult Moina (700-1,000 µm) are longer than newly-hatched brine shrimp (500 µm) and approximately two to three times the length of adult rotifers. Young Moina (less than 400 µm), however, are approximately the same size or only slightly larger than adult rotifers and smaller than newly-hatched brine shrimp. In addition, brine shrimp die quickly in freshwater. As a result, Moina are ideally suited for feeding freshwater fish fry.
Newly-hatched fry of most freshwater fish species can ingest young Moina as their initial food. However, it should be noted that it can be difficult to grade Moina for size. It was found through trials at the UF/IFAS Tropical Aquaculture Laboratory that passing Moina through 500 micron mesh screening tends to fragment the animals to such an extent that they are no longer usable as live food. In aquaria, care must also be taken when determining feeding rates, as Moina can quickly grow too large to be eaten. If these larger Moina become too dense, their “hopping” movements can serve to harass and potentially damage fry.
In Singapore, Moina micrura grown in ponds, fertilized with mostly chicken manure or, less frequently, with pig manure, are used as the sole food for fry of many ornamental tropical fish species, with a 95-99% survival rate to ¾ inch (20 mm) in length quite common. Unfortunately, there is very little information concerning practical mass culture methods of Moina, and the available information is in mimeograph documents, foreign journals or other scarce publications.
Physical and Chemical Requirements
Moina appear in high concentrations in pools, ponds, lakes, ditches, slow-moving streams and swamps where organic material is decomposing. They become especially abundant in temporary water bodies which provide them with suitable conditions for only a brief period.
Moina are generally quite tolerant of poor water quality. They live in water where the amount of dissolved oxygen varies from almost zero to supersaturation. Moina are particularly resistant to changes in the oxygen concentration and often reproduce in large quantities in water bodies strongly polluted with sewage. Species of Moina have been reported to play an important role in the stabilization of sewage in oxidation lagoons.
The ability to survive in oxygen-poor environments is due to their capacity to synthesize hemoglobin. Hemoglobin formation is dependent on the level of dissolved oxygen in the water. The production of hemoglobin may also be caused by high temperature and high population density.
Moina are resistant to extremes in temperature and easily withstand a daily variation of 41-88° F (5-31° C); their optimum temperature is 75-88° F (24-31° C). The high temperature tolerance of Moina is of great advantage for both the commercial fish farmers in the southern U.S. and hobbyists culturing live food at home.
Food Requirements
Moina feed on various groups of bacteria, yeast, phytoplankton and detritus (decaying organic matter). Bacterial and fungal cells rank high in food value. Populations of Moina grow most rapidly in the presence of adequate amounts of bacterial and yeast cells as well as phytoplankton. Moina are one of the few zooplankton which can utilize the blue-green algae Microcystis aeruginosa. Both plant and animal detritus may provide energy for the growth and reproduction of Moina. The food value of detritus depends on its origin and diminishes with the age of the detritus.
Life Cycles of Moina
The reproductive cycle of Moina has both a sexual and asexual phase. Normally, the population consists of all females that are reproducing asexually. Under optimum conditions, Moina reproduce at only 4-7 days of age, with a brood size of 4-22 per female. Broods are produced every 1.5-2.0 days, with most females producing 2-6 broods during their lifetime.
Under adverse environmental conditions, males are produced and sexual reproduction occurs resulting in resting eggs (ephippia), similar to brine shrimp eggs. The stimuli for the switch from asexual to sexual reproduction in populations of Moina is an abrupt reduction in the food supply, resulting in an increase in resting egg production. However, it is advantageous to keep the population well fed and in the asexual mode of reproduction, since fewer progeny are produced with resting eggs.
High population densities of Daphnia can result in a dramatic decrease in reproduction, but this is apparently not the case with Moina. The egg output of Daphnia magna drops sharply at a density as low as 95-115 mature individuals per gallon (25-30/L). The maximum sustained density in cultures of Daphnia reported is 1,900 individuals per gallon (500/L). Moina cultures, however, routinely reach densities of 19,000 individuals per gallon (5,000/L) and are, therefore, better adapted for intensive culture.
A comparison of the production of Daphnia magna and Moina macrocopa cultures fertilized with yeast and ammonium nitrate, showed that the average daily yield of Moina (1.42-1.47 ounces/100 gallons; 106-110 g/m3) is three to four times the daily production of Daphnia (0.33-0.53 ounces/100 gallons; 25-40 g/m3). The daily yield of Moina cultures fed phytoplankton cultured on organic fertilizer have been reported to exceed 5 ounces/100 gallons (375 g/m3).
Nutritional Value of Moina
The nutritional content of Moina varies considerably depending on their age and the type of food they are receiving. Although variable, the protein content of Moina usually averages 50% of the dry weight. Adults normally have a higher fat content than juveniles. The total amount of fat per dry weight is 20-27% for adult females and 4-6% for juveniles.
Procedure for Moina Culture
The batch culture method of producing Moina uses a continuous series of cultures. Briefly, a new culture is started daily in a separate container using the procedures outlined below. When all the fungal, bacterial, and algal cells are consumed, usually about 5-10 days after inoculation, the Moina are completely harvested, and the culture is restarted. This method is particularly applicable when a specific quantity of Moina is needed each day because daily production is much more controlled.
Batch culture is also useful for maintaining pure cultures because there is less chance of the cultures becoming contaminated with competitors (e.g., protozoans, rotifers, copepods) or predators of fish larvae or fry (e.g., Hydra, back-swimmers, diving beetles, dragonfly larvae).
Semi-continuous cultures can be maintained for two months or more by daily partial harvests of Moina, water changes and regular feeding, keeping the population in a state of rapid growth. Eventually, the Moina cultures will fail to respond to additional fertilization. When it is evident that they are not reproducing well, the Moina should be completely harvested and a new culture started.
Moina can be produced either in combination with their food or as separate cultures. Combined culture is the simplest, but production from separate cultures has been reported to be approximately higher.
For separate culture, the phytoplankton tank is positioned so that it can be drained into the Moina culture tank ( Figure 2 ). Production from separate cultures has the disadvantage of requiring additional space for the cultivation of phytoplankton. However, there are advantages of separate culture of Moina and phytoplankton. The advantanges include less chance of contamination, a greater degree of control, and more consistent yield.
Note: Regardless of the culture method, always maintain several Moina cultures to ensure a supply in case of a die-off.
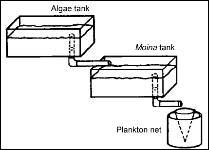 |
| Figure 2. Tank arrangement for the separate culture of Moina and its food. |
Containers
Cultures have been maintained in 10-gallon (38-L) aquaria. However, this volume usually only yields enough Moina for the hobbyist culturing live food. For larger scale and commercial operations, tanks or vats (concrete, stainless steel, plastic or fiberglass) and earthen ponds can be used. Wading pools, plastic sinks, old bathtubs, discarded refrigerator liners and cattle watering troughs also work well. Do not use unpainted metal containers unless they are stainless steel.
In these larger containers, water depth should be no greater than 3 feet (0.9 m). A maximum depth of 16-20 inches (0.4-0.5 m) is probably optimum. The shallow water depth allows good light penetration for photosynthesis by phytoplankton and provides a large surface to volume ratio for oxygen diffusion.
Diffuse light or shade over of the water surface of the Moina culture container is recommended. A greenhouse covered with shade cloth (50-80% light reduction) is ideal. Outdoor cultures should be protected from rain to help stabilize production and screened to prevent entry of predacious aquatic insects.
Containers to be used, whether aquaria, tanks, vats or ponds, need not be particularly clean. However, filamentous algae and predators of fish larvae or fry (e.g., Hydra, back-swimmers, diving beetles, dragonfly larvae) can be especially troublesome in Moina cultures. Tanks can be disinfected with a 30% solution of muriatic acid or by drying in sunlight. Earthen ponds should be drained and sun dried.
Water
Moina are extremely sensitive to pesticides, metals (e.g., copper and zinc, which may be prevalent in municipal or well water), detergents, bleaches and other toxic materials in the water supply. Ensure that toxins are not inadvertently introduced into the culture container. Well water should be aerated for at least two hours. Municipal water should be aerated for at least two days to neutralize the chlorine, or sodium thiosulfate or a commercially available chlorine neutralizer can be added to shorten this process. Natural spring water is ideal. Rain water is also excellent for Moina cultures if it is collected from an area that does not have excessive air pollution. Filtered lake or stream water may also be used.
The optimum water temperature for Moina is 75-88° F (24-31° C). Moina continue to thrive at temperatures in excess of 90° F (32° C) for short periods. However, low temperatures reduce production.
Aeration
Gentle aeration of the Moina pool oxygenates the water, keeps food particles in suspension and increases phytoplankton production; these result in an increase in the number of eggs per female, the proportion of egg-bearing females in the population, and the population density. A small trickle of fresh water into the culture container may also improve production of Moina. Only one or two aquarium air lines are required in culture containers up to 400 gallons (1.5 m3). Extremely small bubbles should be avoided as they can get trapped under the carapace, causing Moina to float at the surface, eventually killing them.
Feeding or Fertilizing
Listed below are some common fertilizer materials and application rates. Try several of these culture media to determine which one works best in your situation. The initial fertilization rates provided are only a starting point and will probably need to be adjusted depending on individual culture conditions.
The following quantity of fertilizer materials should be added initially for each 100 gallons
(379 L) of water. Additional feed or fertilizer, approximately 50-100% of the initial amount, should be added about 5 days later.
- Yeast: 0.3-0.5 ounces (8.5-14.2 g) of baker’s yeast.
- Yeast and mineral fertilizer: 0.3-0.5 ounces (8.5-14.2 g) of yeast, and 0.5 ounces (14.2 g) of ammonium nitrate.
- Alfalfa, bran and yeast: 1.5 ounces (42.5 g) of alfalfa pellets or meal, 1.5 ounces (42.5 g) of wheat or rice bran, and 0.3 ounces (8.5 g) of yeast.
- Cow manure or sewage sludge, bran and yeast: 5 ounces (142 g) of dried manure or sewage sludge, 1.5 ounces (42.5 g) of wheat or rice bran, and 0.3 ounces (8.5 g) of yeast.
- Cow manure or sewage sludge, cotton seed meal and yeast: Use 5 ounces (142 g) of dried manure or sewage sludge, 1.5 ounces (42.5 g) of cotton seed meal and 0.3 ounces (8.5 g) of yeast.
- Horse or cow manure or sewage sludge: Combine 20 ounces (567 g) of dried manure or sewage sludge.
- Chicken or hog manure: Combine 6 ounces (170 g) of dried manure.
- Yeast and spirulina powder: 0.2 ounces (6 g) bakers yeast , 0.1 ounces (3 g) spirulina powder. Add this amount for the first two days, and then every other day until culture is harvested. Note: Add warm water to yeast and spirulina powder and let sit for 30 minutes. Stir and pour contents through a brine shrimp net into the Moina culture. The net will catch un-dissolved yeast and extend the life of the culture.
Organic fertilizers are usually preferred to mineral fertilizers because organic fertilizers provide bacterial and fungal cells and detritus as well as phytoplankton as food for the Moina. This variety of food items more completely meets their nutritional needs, resulting in maximum production. Mineral fertilizers may be used alone, however, they work better in earthen ponds than in tanks or vats.
Fresh manures are preferred because they are richer in organic matter and bacteria. However, some farm animals are given feed additives that control fly larvae in their manure and these may inhibit the production of Moina. Although not absolutely necessary, the manure is frequently dried before use. Commercially available organic fertilizers, such as dehydrated cow manure and sewage sludge, may be used for Moina cultures.
Although manure is widely used to culture Moina, yeast, alfalfa and bran are less objectionable to use and they work well. Activated yeast (baker’s yeast) is readily available from wholesale food distributors in 2-pound (0.9-kg) bags. Bran and alfalfa meal or pellets can be purchased in 50-pound (22.7-kg) bags from livestock feed stores.
Coarse organic materials, such as manure, sewage sludge, hay, bran and oil seed meals, are usually suspended in the water column in mesh bags. Cheese cloth, burlap, muslin, nylon or other relatively loose weave fabrics may be used. Nylon and other synthetic fabrics, however, do not deteriorate in water as do cotton or burlap. For smaller culture containers, nylon stockings work well for this purpose, are inexpensive and readily available. The use of a bag prevents large particles from being a problem when the Moina are harvested and allows greater control of fertilization.
Over-feeding can quickly cause problems in water quality. Regardless of the type of media used, start with small amounts of feed or fertilizer added at frequent intervals and slowly increase the amount used as you gain experience. If fungus occurs in the culture container due to over-fertilization, the bag containing the organic material should be removed from the culture. If fungus persists in large quantities the culture should be discarded and restarted.
Excessively high pH (greater than 9.5), due to a heavy algae bloom and the resulting increase in the proportion of the toxic form of ammonia (un-ionized), may inhibit the production of Moina. The pH of the culture can be adjusted to 7-8 with vinegar (acetic acid).
Inoculating
Use pure live cultures to inoculate. Avoid using animals for inoculation from poor or declining cultures, cultures producing resting eggs, or cultures containing predators of fish larvae or fry. Inoculate with approximately 100 Moina/gallon (25/L). Although a culture can theoretically be started with a single female, always use an adequate number to develop a harvestable population quickly. If fewer are used, the population in the culture will increase more slowly, therefore, the initial quantity of fertilizer or food should be reduced to prevent over-feeding. A greater number used for inoculation reduces the time to harvesting and lessens the chance of contamination by competitors.
Cultures are usually inoculated 24 hours or more after fertilization. However, when yeast is used, Moina can be added to the culture after a few hours of aeration, assuming good water quality and proper temperature. This is because the yeast cells are immediately available to the Moina as food. The small amount of phytoplankton present in the water and digestive tract of the Moina used to inoculate the culture is usually sufficient to initiate a phytoplankton bloom.
Monitoring
The culture should be inspected daily to determine its health. The following observations should be made.
- The health of the culture is determined by stirring the culture, removing 1 tablespoon (15 ml) of the culture, and examining the sample with a 8X- to 10X-hand lens or dissecting scope. Green or brown-red Moina with full intestinal tracts and active movement indicate a healthy culture. Pale Moina with empty digestive tracts or Moina producing resting eggs are indications of suboptimum environmental conditions or insufficient food.
- The population density of Moina is determined by killing the Moina in a 1-teaspoon (3-5/ml) sample with a 70% alcohol solution and counting all Moina in a petri dish with a hand lens or dissecting scope. Cultures ready for harvest should contain 45-75 Moina in the 1-teaspoon sample (3-5/ml). With experience, population density can be estimated visually without the need for counts.
- The food concentration in the culture water, when examined in a clear glass, should appear slightly cloudy and tea colored or green. Clear culture water is an indication of insufficient food. The cultures should be fed or fertilized with approximately 50-100% of the initial quantity whenever the transparency is greater than about 12-16 inches (0.3-0.4 m). This can be determined with a white plastic or metal lid approximately 4 inches (100 mm) in diameter, attached to the end of a yard stick. The depth of transparency is where the disk is just barely visible when lowered into the tank.
- If predators of fish larvae or fry (e.g., Hydra, back-swimmers, diving beetles, dragonfly larvae) are observed, discard the culture and clean and disinfect the tank or pool to avoid contaminating other cultures.
Harvesting
Moina can be harvested by simply dipping out the required number with a brine shrimp net or re-usable coffee filter as they concentrate in “clouds” at the surface. Cultures may also be harvested by draining or siphoning the culture water into a plankton collector equipped with 50- to 150-µm mesh netting net suspended in a container of water. Turn off the aeration and allow the food particles to settle before harvesting. For semi-continuous culture, do not harvest more than 20-25% of the population each day, unless you are restarting the culture. Harvesting by draining the culture tank allows for a partial water exchange, improving water quality. Harvest only small quantities at a time and transfer the Moina to containers with fresh water to keep them alive.
The bottom sediments should be stirred up manually every day following harvest, thoroughly mixing the culture, to re-suspend food particles and prevent anaerobic conditions from developing.
Additional Points
Differences in size, brood production and optimum environmental conditions exist between different species and varieties of Moina. Adjustments will need to be made in the culture technique depending on the particular species or variety you wish to produce.
Additional surfaces in the culture tank may have a positive effect on the production of Moina. For Daphnia, a four-fold increase of surface area, in the form of plastic sheets, has been shown to result in a four-fold increase in the density, biomass and harvest. It is unknown whether this is the result of improved water quality due to nitrifying bacteria on the substrate, a change in the spatial distribution of the Daphnia or improved nutrition.
It may not always be possible to match Moina production to the food demand of the fish fry. Harvested Moina can be kept alive for several days in clean water in a refrigerator. They will resume normal activity when they are again warmed. The nutritional quality of the stored Moina will probably not be optimal because of the period of starvation, so the Moina should be enriched with algae and yeast before feeding them to fish.
Moina can be stored for long periods by freezing in low salinity water (7 ppt, 1.0046 density) or by freeze-drying. Both methods kill the Moina, so adequate circulation is required to keep them in suspension after thawing so they will be available to the fish fry. Frozen and freeze-dried Moina are not as nutritious as live animals and they are not as readily accepted by fish fry. Although freezing or freeze-drying does not significantly alter the nutritional content of Moina, nutrients do leach out rapidly into the water. Nearly all of the enzyme activity is lost within ten minutes after introduction in fresh water. After one hour, all of the free amino acids and many of the bound amino acids are lost.
Footnotes
1. This document is Circular 1054, one of a series from the Department of Fisheries and Aquatic Sciences, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. First published: May 1992. Revised: February 2003. Please visit the EDIS Web Site at http://edis.ifas.ufl.edu.
2. R.W. Rottmann, former Senior Biological Scientist, Department of Fisheries and Aquatic Sciences, Gainesville, and J. Scott Graves, Biological Scientist, Craig Watson, Director, and Roy P.E. Yanong, Assistant Professor, UF/IFAS Tropical Aquaculture Laboratory, Ruskin, FL 33570, Department of Fisheries and Aquatic Sciences, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL 32611.
The Institute of Food and Agricultural Sciences (IFAS) is an Equal Employment Opportunity – Affirmative Action Employer authorized to provide research, educational information and other services only to individuals and institutions that function without regard to race, creed, color, religion, age, disability, sex, sexual orientation, marital status, national origin, political opinions or affiliations. For information on obtaining other extension publications, contact your county Cooperative Extension Service office.
Florida Cooperative Extension Service / Institute of Food and Agricultural Sciences / University of Florida / Larry R. Arrington, Interim Dean
Copyright Information
This document is copyrighted by the University of Florida, Institute of Food and Agricultural Sciences (UF/IFAS) for the people of the State of Florida. UF/IFAS retains all rights under all conventions, but permits free reproduction by all agents and offices of the Cooperative Extension Service and the people of the State of Florida. Permission is granted to others to use these materials in part or in full for educational purposes, provided that full credit is given to the UF/IFAS, citing the publication, its source, and date of publication.